41 fda structured product labels
Structured Product Labeling Resources | FDA Aug 17, 2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. Techmeme Oct 17, 2022 · The essential tech news of the moment. Technology's news site of record. Not for dummies.
FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor verified by FDA. The drug labeling …

Fda structured product labels
DailyMed 15/09/2021 · The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical … What is Structured Product Labeling (SPL)? - Freyr Solutions Structured Product Labeling (SPL) is a standard document issued by Health Level Seven (HL7) to exchange information related to medicinal product and ... MTHSPL (FDA Structured Product Labels) - Synopsis MTHSPL includes drug product and active substance terminology used in Structured Product Labels. MTHSPL contains approximately 158,821 drug products and 21,070 ...
Fda structured product labels. DailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.Instead, these archives have been split into multiple parts. The remainder archive files consist of bulk ingredient labels, vaccine labels, and some labels for medical … MoneyWatch: Financial news, world finance and market news ... Oct 14, 2022 · Get the latest financial news, headlines and analysis from CBS MoneyWatch. What is Structured Product Labeling? | SPL Simplified Apr 22, 2022 ... Structured Product Labeling (SPL) is the FDA's adopted standard for communicating product and quality information. Guidance for Industry - Indexing Structured Product Label - FDA Structured Product Labeling. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER).
Structured Product Labeling Resources | FDA 17/08/2022 · The Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging product and facility information. FDA SPL - Structured Product & Drug Labeling Composition Process Structured Product Labeling (SPL) is a Health Level Seven (HL7) International standard for regulatory guidance documents as a method for communicating product ... MTHSPL (FDA Structured Product Labeling) Source Information The U.S. National Library of Medicine (NLM) produces the Metathesaurus FDA Structured Product Labels (MTHSPL), which is based on the Food and Drug ... FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.)
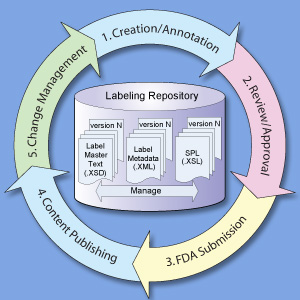
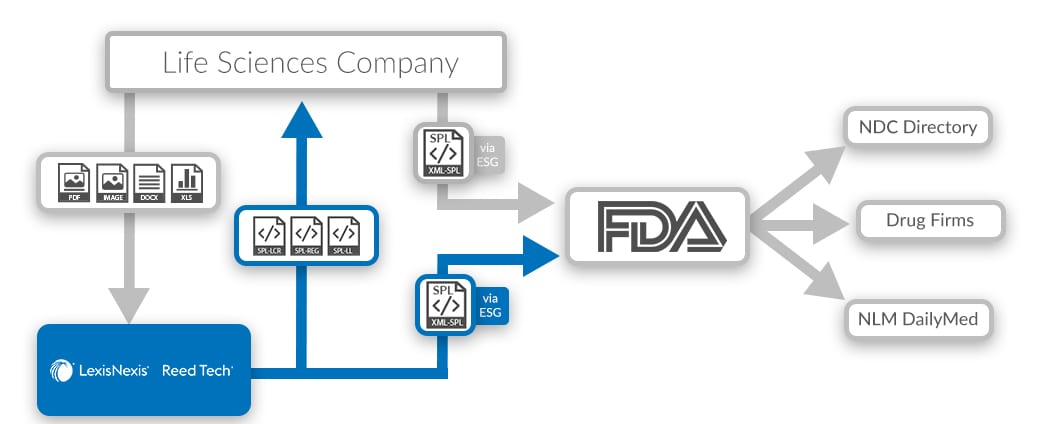
Breast Implants | FDA - U.S. Food and Drug Administration 08/09/2022 · Statement from FDA Principal Deputy Commissioner Amy Abernethy, M.D., Ph.D., and Jeff Shuren, M.D., J.D., director of the FDA's Center for Devices and Radiological Health on FDA's new efforts to ... Reed Tech | Best-In-Class Information-Based Solutions and Services Risk Evaluation and Mitigation Strategies in Structured Product Labeling Format & Additional Pharma Annual Deadlines This webinar recording takes a complete look at the annual deadlines that pharmaceutical companies are required to fulfill to become and remain compliant with the US Food and Drug Administration (FDA), with a special focus on the new REMS in SPL deadline. Structured Product Labeling (SPL) Implementation Guide with ... - FDA Structured Product Labeling (SPL). Implementation Guide with Validation. Procedures. Technical Specifications Document. This Document is incorporated by ... Structured Product Labeling - Wikipedia Structured Product Labeling (SPL) is a Health Level Seven International (HL7) standard which defines the content of human prescription drug labeling in an ...
Structured Product Labeling (SPL) is a document markup ... - openFDA Structured Product Labeling (SPL) is a document markup standard approved by Health Level Seven (HL7) and adopted by FDA as a mechanism for exchanging ...
Devices Guidances | FDA - U.S. Food and Drug Administration Sep 27, 2022 · Contact FDA Follow FDA on Facebook Follow FDA on Twitter View FDA videos on YouTube Subscribe to FDA RSS feeds FDA Homepage Contact Number 1-888-INFO-FDA (1-888-463-6332)
DailyMed - Download All Drug Labels Full Releases. Warning: The full human prescription and OTC archive files, dm_spl_release_human_rx.zip and dm_spl_release_human_otc.zip, are no longer available due to size considerations.
NSDE | FDA - U.S. Food and Drug Administration Mar 31, 2022 · With the exception of the billing unit data in the NSDE document, this file is generated from SPL documents sent to FDA for inclusion in the FDA Online Label Repository at labels.fda.gov.
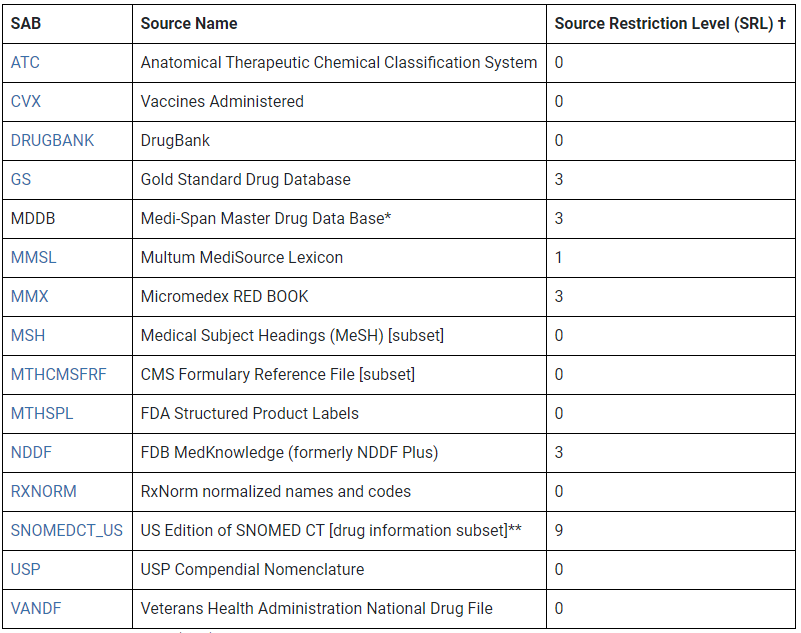
MTHSPL (FDA Structured Product Labels) - Synopsis MTHSPL includes drug product and active substance terminology used in Structured Product Labels. MTHSPL contains approximately 158,821 drug products and 21,070 ...
What is Structured Product Labeling (SPL)? - Freyr Solutions Structured Product Labeling (SPL) is a standard document issued by Health Level Seven (HL7) to exchange information related to medicinal product and ...
DailyMed 15/09/2021 · The National Library of Medicine (NLM)’s DailyMed searchable database provides the most recent labeling submitted to the Food and Drug Administration (FDA) by companies and currently in use (i.e., "in use" labeling). DailyMed contains labeling for prescription and nonprescription drugs for human and animal use, and for additional products such as medical …




.png.aspx)









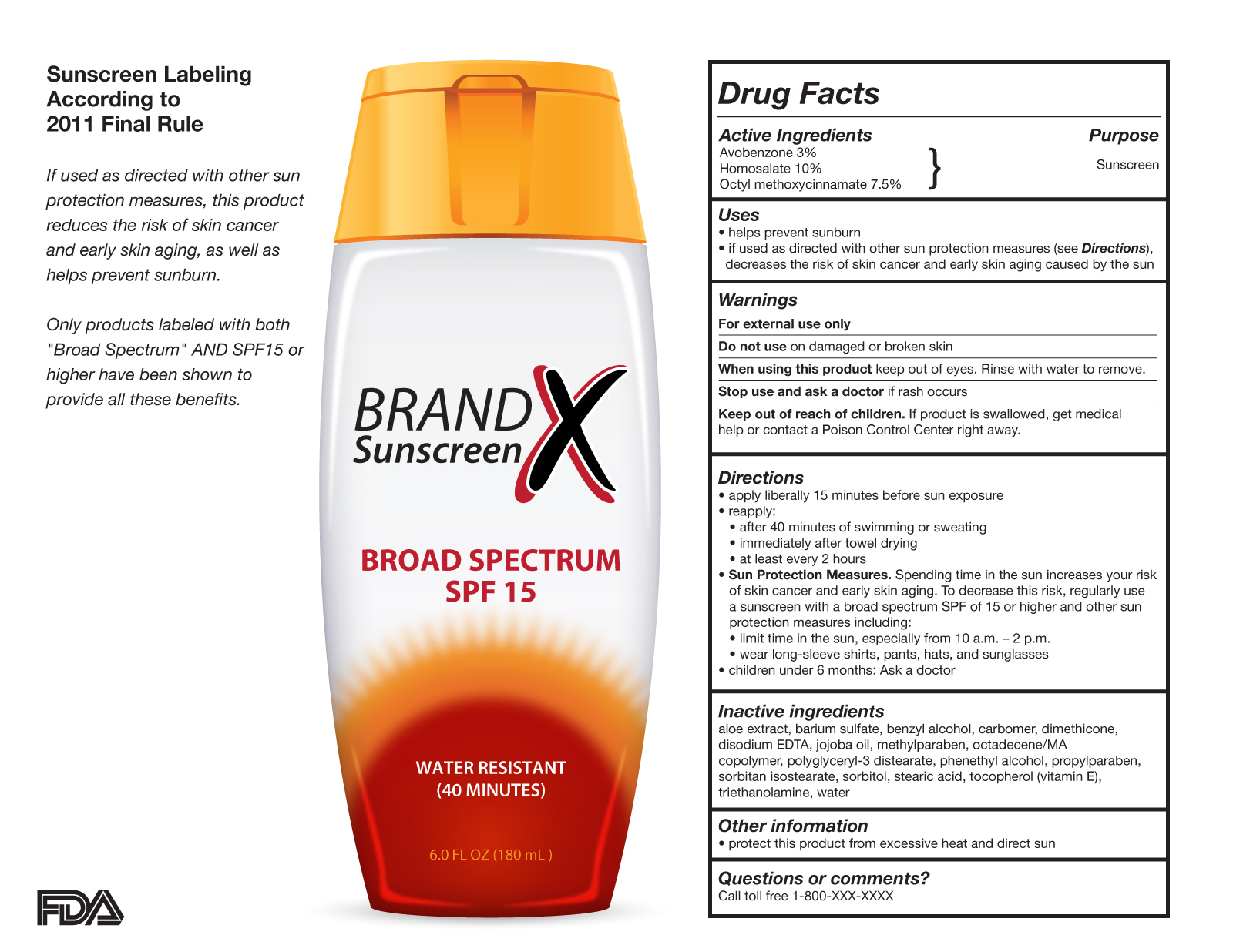



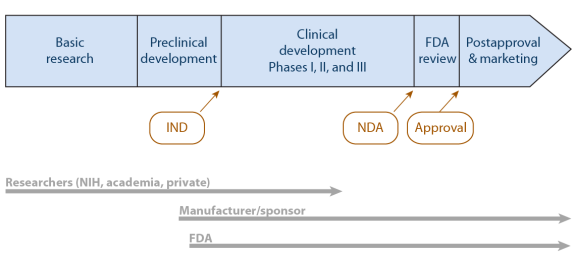
![Food Labeling 101 - FDA Regulations Guide [2022] | Artwork Flow](https://global-uploads.webflow.com/5f59aa263c234bb74025de57/5fa4f81b6e365340866e0eeb_Inner-Images-4.jpg)


Post a Comment for "41 fda structured product labels"